Who We Are
EpimAb Biotherapeutics Inc is a clinical stage biopharmaceutical company specializing in the development of multispecific antibodies. Utilizing our broad range of in-house research and technology capabilities, including the proprietary FIT-Ig® (Fabs-In-Tandem Immunoglobulin) and MAT-Fab (Monovalent Asymmetric Tandem Fab) bispecific platforms, EpimAb is generating and globally advancing a unique pipeline of transformative preclinical and clinical assets that aim to benefit cancer patients.


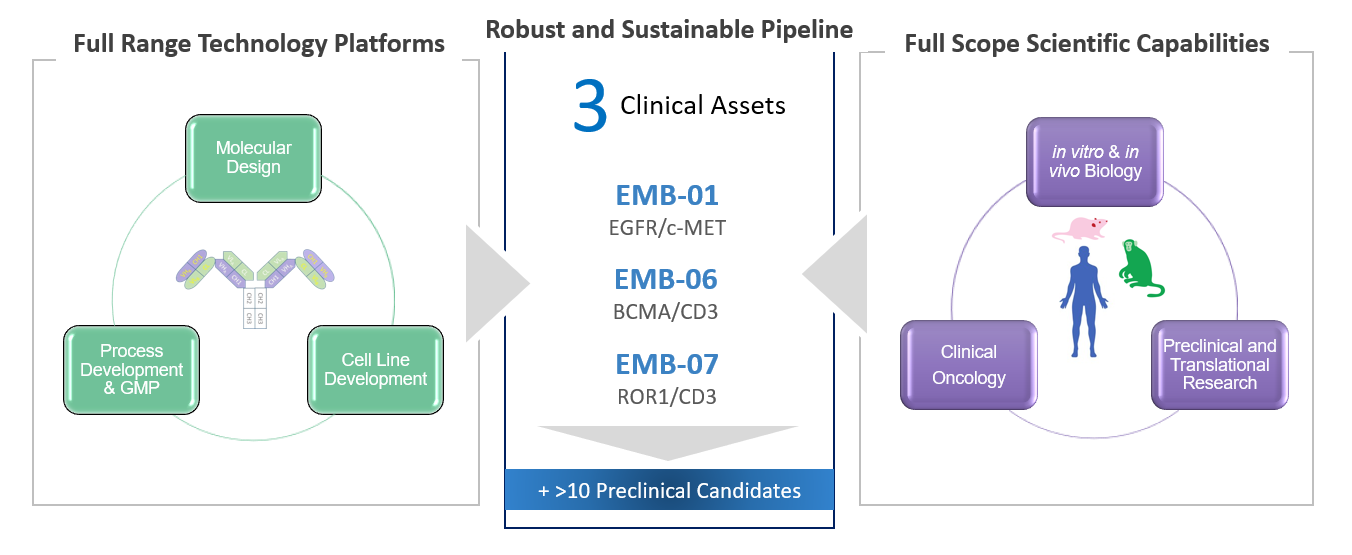
Promising Pipeline Derived From In-House Research
Preclinical and clinical assets address global unmet medical needs
Sustainable first-/best-in-class pipeline with 3 candidates in the clinic
Novel and Proprietary Bispecific Antibody Technology Platform
World-class innovative BsAb platform with strong IP
Rapid and proven bispecific antibody development process that is comparable to that for typical monoclonal antibodies
Seasoned and Well-rounded Management Team with Global Track Records
Dr. Chengbin Wu is a top expert in the BsAb area with a well-established track record
Team includes other industry veterans with complementary experiences from early discovery, clinical development, CMC, to business development and financing
EpimAb Aims to Redefine Bispecific Antibodies (BsAb)
EpimAb Milestone

Join the dynamic team at EpimAb to maximize your potential
and explore cutting-edge science
and explore cutting-edge science














